Technetium(VII) oxide
Appearance
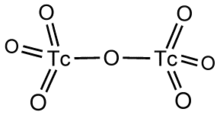 Structural formula of technetium(VII) oxide.
| |
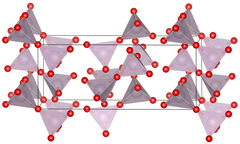 Unit cell of technetium(VII) oxide.
| |
| Names | |
|---|---|
| IUPAC name
Technetium(VII) oxide
| |
| Other names
Technetium heptoxide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Tc2O7 | |
| Molar mass | 307.810 g/mol |
| Appearance | light yellow solid |
| Density | 3.5 g/cm3 |
| Melting point | 119.5 °C (247.1 °F; 392.6 K) |
| Boiling point | 310.6 °C (591.1 °F; 583.8 K) |
| hydrolysis to HTcO4 | |
| −40.0·10−6 cm3/mol | |
| Structure[1] | |
| Primitive orthorhombic | |
| Pbca, No. 61 | |
a = 1375.6 pm, b = 743.9 pm, c = 561.7 pm[1]
| |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
radioactive |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Technetium(VII) oxide is the chemical compound with the formula Tc2O7. This yellow volatile solid is a rare example of a molecular binary metal oxide, the other examples being RuO4, OsO4, and the unstable Mn2O7. It adopts a centrosymmetric corner-shared bi-tetrahedral structure in which the terminal and bridging Tc−O bonds are 167pm and 184 pm respectively and the Tc−O−Tc angle is 180°.[2]
Technetium(VII) oxide is prepared by the oxidation of technetium at 450–500 °C:[3]
- 4 Tc + 7 O2 → 2 Tc2O7
It is the anhydride of pertechnetic acid and the precursor to sodium pertechnetate:
- Tc2O7 + 2 H2O → 2 HTcO4
- Tc2O7 + 2 NaOH → 2 NaTcO4 + H2O
References
[edit]- ^ a b Krebs, Bernt (1971). "Technetium(VII)-oxid: Ein Übergangsmetalloxid mit Molekülstruktur im festen Zustand". Zeitschrift für anorganische und allgemeine Chemie. 380 (2): 146–159. doi:10.1002/zaac.19713800205.
- ^ Krebs, Bernt (1969). "Technetium(VII)-oxid: Ein Übergangsmetalloxid mit Molekülstruktur im festen Zustand". Angewandte Chemie. 81 (9): 328–329. Bibcode:1969AngCh..81..328K. doi:10.1002/ange.19690810905.
- ^ Herrell, A. Y.; Busey, R. H.; Gayer, K. H. (1977). Technetium(VII) Oxide, in Inorganic Syntheses. Vol. XVII. pp. 155–158. doi:10.1002/9780470132487.ch41. ISBN 0-07-044327-0.
